Research remains an essential part of cancer care. In this blog, we will provide information about recent updates or new approvals as it applies to breast cancer written by Dr. Sharon Wilks.
Oserdu, an Oral SERD, was recently FDA approved for Recurrent Metastatic Hormone Receptor Positive Breast cancer with ESR1 mutations.
What does this mean for women with breast cancer?
We have a new treatment option for women with metastatic breast cancer. Oserdu® is a new oral option for patients with metastatic breast cancer. In addition to having yet another option in care, what does this drug’s availability allow us to understand about breast cancer and moving forward with research?
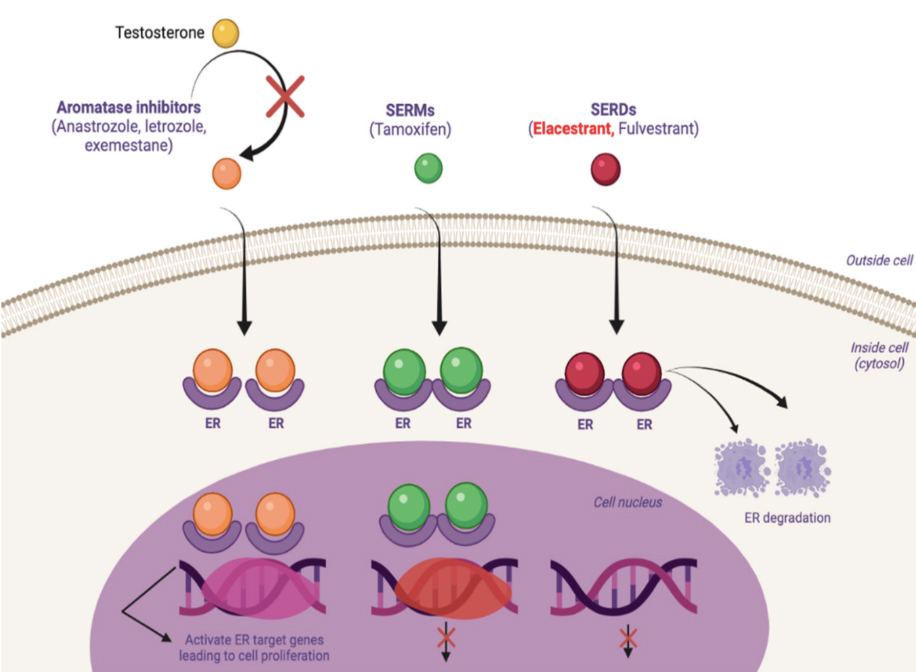
First, what is Oserdu®? It is the brand name of an agent that has been in clinical research for a while, referred to as Elascestrant. This agent is known as a SERD, or Selective Estrogen Receptor Degrader. These drugs bind to the estrogen receptor which leads to the receptor to be down regulated. When this occurs, this leads to suppression of the estrogen signaling which allows for control/reduction of tumor growth and proliferation.
Until recently, Fulvestrant which is given as an injection monthly after an initial load, was the only agent in the SERD- class FDA approved for use in patients with Hormone Receptor positive breast cancer. Now we have our first oral SERD for treatment for these individuals with recurrent breast cancer.
In patients with metastatic hormone receptor positive breast cancer with prior exposure to hormone blockade, specifically Aromatase Inhibitors (AIs), ESR1 mutations are reported to occur in ~40%. When this mutation is present, this leads to an alteration in the ligand-binding site on the estrogen receptor. When this happens, diminishing or removing estrogen concentration does not lead to stoppage of tumor growth whereas in systems without this alteration, the success of AI therapy and Tamoxifen has occurred due to the need of Estrogen to stimulate this signaling pathway for tumor growth: having ESR1 mutations leads to autonomous cancer growth whether estrogen is present or not. Though Fulvestrant has benefit as a SERD, many reports have suggested that it is not as effective for control of estrogen signaling with ESR1 mutations are present.
Another important understanding about ESR1 mutations in this setting is that they form over time and are acquired. As mentioned previously, they are more likely to develop in breast tumors after prior exposure of patients to Endocrine blockade therapy with more risk of development in patients who have received prior AI therapy. When tumors are sampled in patients with limited to no prior exposure to endocrine therapy, the incidence is about 2% overall in early stage settings and may be as high as 4% in the metastatic setting for those not previously exposed to these hormone blocking agents but then rises to 30-40% after endocrine exposure. This is an example of how prior therapy can influence tumor resistance to subsequent treatments: Tumors that once may have been controlled with use of endocrine blockade or hormone suppression become resistant and still grow and have metastatic potential. This discovery augmented the need for alternative ways to fight this more resistant tumor.
The recent approval of the first oral SERD, Oserdu® (Elascestrant) in January 2023 was based upon results of a Phase 3 study known as the EMERALD study. This study included 478 patients with Hormone Receptor positive HER 2 Neu-low expressing breast cancer who were randomly assigned to standard hormone blockade agents including Fulvestrant or Aromatase Inhibitors or Elacestrant®. In this study, 100% of these patients had already received prior CDK4/6 inhibitor (Ibrance®/Palbociclib, Kisqali®/ Ribociclib or Verzenio®/Abemaciclib). In a recent update of the EMERALD study (post-hoc analysis) at the San Antonio Breast Cancer Symposium (SABCS) in December 2022, it was discovered that those individuals who experienced long disease control (of at least 6 or more months with use of a CDK4/6 inhibitor) were the individuals that appeared to benefit from subsequent use of Elacestrant. This observation of the duration of response with use of prior CDK4/6 inhibition suggested that patients were likely to still have tumors that were estrogen receptor sensitive. This means that though estrogen blockade was ineffective, estrogen signaling was still a key driver of tumor growth and use of a different measure to stop tumor proliferation could still be effective. In settings where the duration of disease control was shorter with prior CDK4/6 inhibitor therapy (data presented @ SABCS 12/2022 by Dr. Virginia Kaklamani et al) with progression free survival < 6 months, suggesting an endocrine insensitive process, there was less benefit with use of this new oral SERD in comparison to standard treatments suggesting this may be a cohort that would benefit from other strategies than hormone signaling blockade.
Also, amongst patients with ESR1 mutations, there was a statistically significant progression free survival (PFS) improvement amongst patients who received Elacestrant -approximately nine months PFS compared to those who received standard endocrine blockade (median PFS with the latter was about two months). Approximately, 45% of patients treated with Elacestrant experienced less progression and death.
So, what has this new treatment option provided us? Now we have an effective treatment, the first, for patients with ESR1 mutations in hormone receptor positive breast cancer. This treatment has allowed us to see that use of a specific targeted therapy can provide clinically meaningful treatment. This option comes in a pill form which allows avoidance of monthly injections and monthly visits to the doctors office. Most patients tolerate treatment well though there is a risk of nausea, muscle aches, changes in cholesterol studies and diarrhea and Anemia.
Can we improve upon this success? The interest is to obviously improve upon survival rates. Many are studying combinations of SERD with other existing therapies, such as combining oral SERDS with CDK4/6 inhibitors, use of oral SERDs with mTOR inhibitors such as Everolimus, etc.
Hormone receptor positive breast cancer is the most common form of breast cancer and recent data suggests that early-stage patients have a 50% risk of late recurrences after year five post diagnosis and so, introduction of new agents like SERDs in the early stage setting may provide better tumor control and those studies are already in place.
We at NEXT Oncology believe in new options in research to improve survival for men and women with cancers that reoccur. When you or someone you know face an option in treatment of cancer, please consider participating in a research clinical trial, it may save your life. Thanks to the special women in the EMERALD study, we now have a new pill option to fight recurrent breast cancer.