Presented at SABCS 2022 by Len-Shu Lu from the National Taiwan University Hospital, Taipei Taiwan.
Research remains an essential part of cancer care. In this blog, we will provide information about recent updates or new approvals as it applies to breast cancer written by Dr. Sharon Wilks.
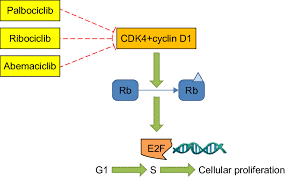
The use of CDK4/6 inhibitors has emerged as a valuable treatment in the first line setting in patients with hormone receptor (Estrogen Receptor/ER and/or Progesterone Receptor/PR or both) breast cancer. Prior to the availability of these agents, use of Endocrine blockade – typically Tamoxifen for premenopausal women and Aromatase Inhibitors in postmenopausal – was the standard approach. Survival rates demonstrated the use of these endocrine blockade agents alone but typically reserved for women with minimal burden of cancer, for example in the setting where there might a few areas of bone metastases or skin only disease or very, very low burden of disease such as small nodules in the lungs. As the availability of CDK4/6 inhibitors emerged, adding these agents (Palbociclib/Ibrance®, Ribociclib/Kisqali®, and Abemaciclib/Verzenio®) was done. Numerous reports in recent years have shown that these additional agents are generally well-tolerated and have improved survival rates significantly. Many patients can experience meaningful survival rates for many years with this combination but there still has been some reluctance to use this combination in patients with more aggressive settings, such as rapidly progressive liver and lung metastases, situations where there is a lot of disease-multiple sites of cancer, etc. In those settings where disease amount is large and brisk growth, most clinicians have still continued to choose chemotherapy to aggressively control disease rapidly – particularly in patients who are young/premenopausal where studies have shown in the past rapid growth like this have led to rapid demise and death.
Recently, studies have shown that it may be effective and improve survival rates to use CDK4/6 inhibitors + Endocrine blockade but in the premenopausal setting, there still was some uncertainty about the efficacy of this approach in high tumor burden states in younger women.
In the current study of 222 patients, this trial looked at Ribociclib. There have been numerous studies showing its value and good tolerance in young women who are still premenopausal (typically ovarian function suppression therapy such as Lupron® and Zoladex®/Goserelin have been used instead of Tamoxifen and when used, an Aromatase Inhibitor has been added). Furthermore, in many of the so called MONALEESA studies (three Phase III studies, MONALEESA 2, 3 & 7), we now know that use of Ribociclib has led to improvement of overall survival in the metastatic setting. It made sense to use this as the experimental arm agent and so the study design included Ribociclib 600 mg orally daily for three weeks on and one week off schedule in combination with Letrozole or Anastrazole (Aromatase Inhibitors) + Goserelin versus treatments that are commonly used as traditional forms of Chemotherapy (CT): Docetaxel + Capecitabine or Paclitaxel + Gemcitabine or Vinorelbine + Capecitabine). I will add that combination CT is normally considered rather than monotherapy in the aggressive setting in hopes of more rapid onset of response and disease control. Again, note that inclusion criteria to be eligible included young women with aggressive disease HR (defined by having an ER >/=10%) positive and HER 2 Neu Negative breast cancer: symptomatic visceral (liver and lung) metastases, rapid disease progression or impending visceral compromise or markedly symptomatic non-visceral disease. Tumor assessments occurred every six weeks for the first 12 weeks and then every eight weeks for the next 32 weeks and then every 12 weeks.
Basic characteristics of the study patient population included greater than 60-70% of subjects were under or at 40 years of age, 29-34% had grade 3 disease, less than 60% were individuals with de novo (stage 4 breast cancer at original diagnosis in distinction of those with cancers that had been early stage and then progressed or recurred-arguably. These patients are expected to have more chemotherapy sensitivity and responsiveness than those who relapse after prior chemotherapy exposure) and at least 50% had either lung or liver metastases (or both). Progression Free Survival/PFS (refers to the interval of time to progression after treatment was started) was nearly doubled favoring the group given Ribociclib+ AI and Goserelin with 24 months prior to progression compared to 12.3 months in the CT group. ORR (overall response rates) & CBR (clinical benefit rates) were at least as good but better than CT-overall rates were at least 60% confirming the high response seen with these treatments in these patients. Also, median time of response (TOR) was similar with 4.9 months versus 3.2 months in the Ribociclib + ET versus CT groups. Tolerance to therapy was superior with the Ribociclib group compared to CT also. Use of the Ribociclib based therapy led to less onset of Nausea, hair loss and fatigue. It is important to know that use of Ribociclib does lead to lower blood counts particularly low White Blood cells (WBC) and Neutropenia (this was more commonly reported than in the CT group – 82% versus 49% with Ribociclib and CT respectively) and liver enzyme changes despite its non-CT approach.
This Phase 2 randomized study has now provided more evidence for consideration of a non-CT approach in patients with metastatic HR+/HER 2 NEG (Low-expressing breast cancer).
I think many of the audience that attended SABCS 2022 who listened to this talk were impressed with this information which offers more support to consider non-CT approaches in the first line setting even with rapidly progressive and aggressive presentations for patients like those included in this study.
As you or someone you know considers new treatment options, please consider research for your care. We at NEXT Oncology encourage research participation when treatment for cancer is necessary.
