Research remains an essential part of cancer care. In this blog, we will provide information about recent updates or new approvals as it applies to breast cancer written by Dr. Sharon Wilks.
For years, breast cancer treatments have been influenced by the information obtained at the time of the original biopsy of the breast cancer. When someone is diagnosed with breast cancer, typically an image guided biopsy is carried out and this material that is collected is reviewed by a pathologist to verify if the person has breast cancer and unique to this diagnosis, additional testing is done to determine the status of the presence or absence of the Estrogen Receptor (ER), Progesterone Receptor (PR) and HER (Human Epidermal Growth Factor) 2 NEU. Thus, if the ER and/or PR is present, the tumor is considered Hormone (Receptor) positive. The absence of all three receptors is consistent with a diagnosis of Triple Negative Breast cancer (TNBC).
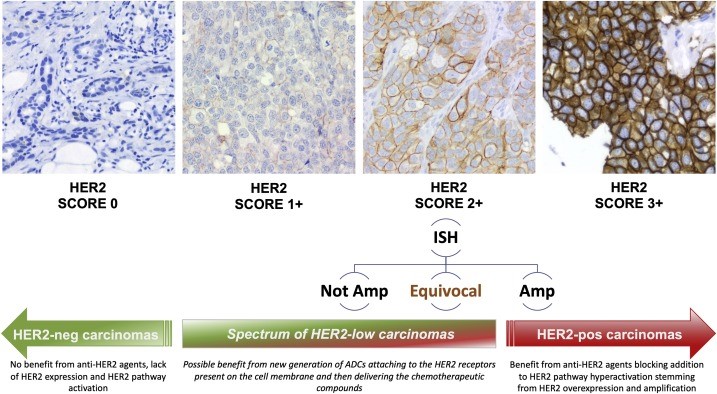
Determining the HER 2 Neu status on tumors is a little bit more complex. Typically, the HER 2 NEU is tested by an ImmunoHistochemical (IHC) stain. There can be four different levels (0-4) of stain intensity with this test. An IHC of 0 shows no staining, an IHC of 1+ has very light staining, and IHC of 2+ has moderate staining and IHC of 3+ has very intense staining. For tumors with IHC 2+, typically another test, FISH (Fluorescence In Situ Hybridization) determines if there are extra copies of the HER 2 gene. Two results come from this analysis: FISH negative (indicative or normal expression of HER 2 genes) and FISH positive (consistent with an increased number of copies of the HER 2 gene or Overexpression).
There have been several drugs that are approved that target the HER 2 Neu receptor. Studies with these drugs (e.g. Trastuzumab/Herceptin®, Lapatinib/Tykerb®, TDM-1/Kadcyla®, Neratinib/Nerlynx®, Tucatinib/Tukysa®, etc.) have shown benefit only in the patient with a HER 2 NEU positive or HER 2 NEU overexpressing tumor.
Recently, a drug Trastuzumab Deruxtecan/Enhertu® has been shown to induce significant disease control for patients with recurrent HER 2 NEU Overexpressing breast cancer but early work with this agent in the Phase I setting had suggested that it also may be beneficial in HER 2 NEU Negative or Low expressing tumors (e.g. HER 2 NEU 1+ and 2 +/FISH negative). These observations prompted a study that was presented by Dr. Shanu Modi at the recent ASCO 2022: Trastuzumab Deruxtecan(T-Dxd) versus Treatment of Physicians Choice (TPC) in patients with HER 2-Low, Unresectable and/or Metastatic Breast cancer (MBC), Results of Destiny Breast 04, a Randomized, Phase 3 study.
In this presentation, which was subsequently published, significant improvements in Progression Free Survival (PFS) and Overall Survival (OS) were reported in the group that received the Enhertu® compared with drugs that were previously standard of care in patients with breast cancers that were either HER 2 Neu 1+ by IHC or HER 2 NEU 2+ with a FISH negative result. Results from this study have led to a recent FDA approval for Enhertu for individuals with MBC with low HER 2 NEU expression.
These findings have now made us rethink the concept of HER 2 NEU negative versus positive and instead, we consider description of HER 2 NEU over/high-expression breast cancer (IHC of 3+ and or FISH positive) versus HER 2 NEU low expressing breast cancer (IHC of 1 or 2+, the latter must have a tumor that tests FISH negative). This new approval has offered so much promise and hope for breast cancer outcomes, but it is anticipated that we still will need additional novel treatments to fight this form of breast cancer.
Recently, the NEXT Oncology clinic opened a new trial that combines a novel combination of agents that is being looked at for patients with MBC with HER 2 NEU high and low expressing breast cancer. Why do we need another agent, you might ask? Unfortunately, it has been shown that progression still occurs with recent approved drugs, and we need better ways to fight cancer. Another reason that we need this research is that although approved therapies may be effective, some patients find it too difficult to tolerate them and unfortunately, may have to stop use of these agents. The new study at NEXT Oncology involves the use of a drug that is remarkably engineered and designed to introduce novel components that is anticipated to provide another option in care for women facing this terrible disease. As always, please consider research options if offered. Research can offer more effective and potentially more tolerable ways to fight cancer.

