Research remains an essential part of cancer care. In this blog, we will provide information about recent updates or new approvals as it applies to breast cancer written by Dr. Sharon Wilks.
Educational Symposium on ADCs @ December 2022 SABCS.
Bioengineering & Mechanism of Action of Antibody Drug Conjugates (ADC)
Presentation from Puja Sapra, PhD with SVP Biologics Engineering and Targeted Delivery, Astra Zeneca
ADCs in Breast Oncology: Present & Future
Presentation provided by Dr. Shanu Modi, MD, Memorial Sloan Kettering.
These two outstanding talks at the recent SABCS in December 2022 provided a comprehensive view of the state-of-the-art research as it relates to the utilization of these new agents referred to as Antibody Drug Conjugates (ADCs) in the management of all forms of breast cancer. In the last few years, these agents have found a place in the therapeutic armamentarium for women and men facing recurrent breast cancer. These new agents have provided new hope in disease control. ADCs have been on the market for a variety of uses in cancer care since the 2000s but from 2013 to the present, we now have a couple of these agents for use in breast cancer.
The first generation drug used was Kadcyla® also known as T-DM-1 (T= Trastuzumab) or Trastuzumab Emtansine approved for HER2 Neu positive or Overexpressing/High breast cancer (defined as IHC 3 + staining or Flourescence In Situ/FISH positive). The next generation of agents included Enhertu® also known as T-DXd (Trastuzumab Deruxtecan) was approved by the FDA originally in 2019. TDM-1 was originally only approved in the metastatic setting but obtained another approval later after an important trial known as the KATHERINE trial showed meaningful benefit in patients that had undergone chemotherapy in the neoadjuvant/preoperative setting but were found at surgery to have residual disease. Use of this agent in place of Herceptin® (Trasutuzumab) showed significant (50% improvement) in Disease Free Survival in patients with HER 2 Neu positive. TDXd was originally approved in the third line in 2019 for HER 2 Neu positive breast cancer. More recently, the NCCN recommended its use in second line setting for patients with HER 2 Neu Overexpressing Breast cancer. Its latest FDA approval (August 2022) was based on a remarkable study known as DESTINY Breast 04(BO4) presented at the 2022 ASCO by Dr. Modi for use in patients with what is now referred to as HER 2 Neu Low Expressing (defined as HER 2 Neu 1+ by Immunohistochemical/IHC staining or HER 2Neu 2+ by IHC but this group must have breast tumors that are Fluorescence In Situ/FISH negative for HER 2 Neu) Breast cancer.
This last approval has caused a paradigm shift in management of patients with Hormone receptor positive (and to some degree Hormone receptor Negative) breast cancer. This application now allows for more individuals with recurrent breast cancer to live longer than ever reported previously.
The third ADC approved in breast cancer is known as Trodelvy® (its generic name is Sacituzumab Govetican) and it targets an antigen known as TROP-2 (this antigen is heavily expressed in the majority of breast cancers and its presence confers a poor prognosis). This agent was FDA approved for second line use in patients with Triple Negative breast cancer in 2020 based upon a study known as the ASCENT study, a Phase 3 study that compared Trodelvy® versus Treatment of Physician choice (drugs like Capecitabine, Eribulin, Gemcitabine, Vinorelbine-drugs with established benefit in patients with advanced forms of breast cancer). More recently, a study known as TROPICS-02 addressed the use of this agent, Trodelvy® in patients with Hormone Receptor positive but HER2 Neu negative (included a cohort of HER 2 Neu 0 expressing tumors). This study demonstrated benefit in PFS (Progression Free Survival) & OS (Overall survival) in this group of patients too.
As one might anticipate, T-DXd which is a second-generation ADC has shown remarkable improvement in outcomes for breast cancer control and survival when compared to TDM-1. At SABCS 2022, a study known as DESTINY B03 was updated. This study looked at patients with HER 2 Neu overexpressing breast tumors that had previously been treated with Trastuzumab & Taxane based therapy in the advanced setting and randomized these individuals to use T-DXd to T-DM1 every 3 weeks and notably/remarkably those individuals who received T-DXd experienced a 4-fold improvement in Progression Free Survival (PFS) when compared to T-DM-1 (median PFS was 28.8 months versus 6.8 months favoring the use of T-DXd when compared to T-DM-1). There was also confirmation of a statistically significant Overall Survival (OS) with a p value of 0.0037 and Hazard ratio of 0.64 in favor of the group treated with T-DXd.
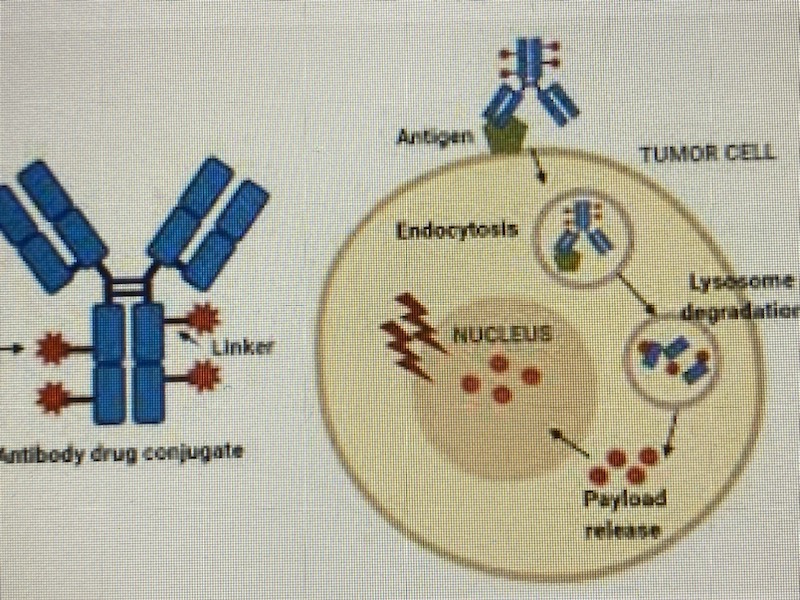
Though we would intuitively anticipate a second-generation drug would be better than a first generation drug, in the presentations in this educational forum, we gathered more understanding about the structure of ADCs to explain the reasons for this improved efficacy. We learned that every component of the ADC matters: the antibody that finds the target/cancer cell, the linker that attaches the antibody to the so-called payload and the payload.
In the wonderful review of Antibody Drug Conjugates (ADCs) given by Dr. Sapra, she reviewed the History of ADC Development, the different components of ADCs and learnings based upon the design of the components. She also shared a comparison & contrast of HER 2-ADCs and lastly, the future perspectives regarding the use and study of these relatively new/novel agents in cancer care.
How Do ADCs Work?
An Antibody Drug Conjugate (ADC) is an antibody that is designed against an antigen that is expressed on cancer cells or which is overexpressed on cancer cells. The Antibody portion facilitates the binding of the agent/moiety to the targeted antigen and then the entire unit is internalized by receptor mediated endocytosis. Inherent antibody mediated ADCC activity also occurs.
As previously indicated, an ADC is a composite of an ANTIBODY, which facilitates binding of the cancer target that then is internalized and facilitates immune effector changes, a LINKER which provide a bond of the antibody component to the drug payload and the PAYLOAD which is the component that that via numerous mechanisms is felt to be the active ingredient that stops cancer growth. The proposed mechanism of action for the ADCs to date has been the concept of binding to only the cells that contain the target and then upon binding of the antibody to the target, internalization of the composite (drug moiety) into the targeted cancer cell occurs. Once internalized, natural enzymes/lysosomes break apart the components with cleavage of the Linker component and then with release of the Payload internally, destruction of cancer cells through effects on mitosis that leads to cell death takes place. Based upon more recent observations in study, in addition to this targeted effect, the active cytotoxic component also may permeate/penetrate outside of the targeted cancer cell membrane and affect many neighboring cancer cells that may not have the same expression of the target and these cells can also be destroyed via a new concept of a “bystander effect” thus non-targeted cancer cells also can experience tumoricidal effects.
Why do some ADCs offer more benefit than others? Though we have 12 ADCs approved for a variety of cancers to date, at least 55 other ADCs studied have failed either due to an excess toxicity or due to limited effectiveness. Why?
It appears that ALL of the Components of the ADC matters:
It appears that the antibody used to find the target matters, at present, in breast cancer, to date, we have drugs that target HER 2 Neu and TROP-2. One problem with the Target is that most tumors are heterogeneous and that a target that might seem appealing has then gone on to fail in disease control due to the lack of binding of the ADC in a meaningful way to facilitate drug/payload delivery.
Research is ongoing to look at other targets. Some receptors or antigens appear to have different internalization kinetics so that it may take too much time for the drug to become internalized for ultimate drug delivery and cell kill. Another aspect that appears relevant in cancer control is the drug to antibody ratio: for T-DM-1, the drug to antibody ratio (DAR) is 3.5:1 while T-DXd has a DAR of 8:1. The value of potency also may depend upon the time that the drug is released internally. In the setting of the HER 2 neu overexpressing receptor, if the drug is not internalized quickly, the receptor has the potential to recycle and thus, the potency of the drug is lost. Dr. Sapra explained that with the HER 2 receptor, recycling of the receptor occurs quickly: in 5 minutes, 50% of the receptor is recycled and by 30 minutes, the HER 2 Neu receptor is recycled 90%. If this receptor is restored to its prior function, this recycling of the receptor abrogates the effects of any drug that may have been internalized.
Another key factor is the time of release of the drug by natural lysosymes once the drug is internalized. It appears that payload release is enhanced if the Linker is cleavable. Though efficacy has been demonstrated in drugs with non-cleavable linkers, the technology of T-DXd includes a cleavable linker that may mitigate the time that is necessary for drug/payload release. Furthermore, this cleavable aspect may facilitate the drugs permeability to neighboring cells allowing a drug like T-DXd to have broader efficacy in tumors that don’t contain as much of the antigen or target such as the case of its benefit in the HER 2 neu Low-expressing breast cancers. In Dr. Modis’ review, she reminded us that we have even seen benefit of T-DXd in HER 2 Neu 0 states based upon the Phase 2 study known as DAISY where investigators showed a similar benefit of T-DXd in their cohort 3 who had, by current definition, patients with tumors with no/0 HER 2 Neu expression – so permeability of the ADC also may be key.
We have seen success but what are some of the unmet needs and what is ongoing research addressing?
One important issue of concern is that when ADCs were first created, they were felt to have the potential to be better than traditional cytotoxic chemotherapy agents in regard to their designed specificity for the tumor cell. The hope was use of these ADCs would lead to less toxic effects on normal cells and avoid common effects like nausea, diarrhea, low blood counts, alopecia, etc.
Though we are happy to have the current ADCs available, these agents still can lead to toxic effects on patients. Currently, we are seeing 10-15% rates of Interstitial Lung Disease (ILD) reported in more contemporary studies with T-DXd-less fatalities have been reported to date, but stoppage of this therapy has been necessary in some due to this toxicity manifested as shortness of breath and cough. We also see reports of diarrhea, neutropenia, thrombocytopenia, liver enzyme changes, nausea and other adverse effects with these agents. This reminds us that ADCs are not truly targeted therapies. Dr. Sapra explained that the conjugation chemistry of the linker to antibodies and the payload to date have not been perfect: with some ADCs studied, the payload was released into the circulation prior to antibody binding of the target and some non-targeted cells have picked up the ADC as well. The technology of the Linker component clearly matters as well. We still need improved ways to enhance tumor cell kill without toxicity.
Another research strategy that is being explored is to use other agents or treatments aside from cytotoxic drugs as the payload including use of oligonucleotides, radionuclides, immunomodulators and other targeted therapies for tumor cells.
Though we have some biomarker information for T-DXd for HER 2 high or low expression (though remember there was activity reported in HER 2 Neu 0 in the Phase 2 DAISY study), at SABCS 2022, Dr. Hope Rugo showed that Trodelvy® works similarly in patients with or without TROP-2 expression. These observations along with other ongoing research reminds us that we need biomarkers to predict for activity of the existing and future ADCs to determine which patient populations would benefit.
So as usual, the recent SABCS meeting delivered us new options in improvement in breast cancer care. However, this review reminds us of the importance of ongoing research that is critical-are there other payloads that may offer better success and duration of benefit with less toxicity? Can we use these agents in earlier phases of treatment (research for Neoadjuvant and Advjuvant use for T-DXd is ongoing)? Can we combine ADCs safely with other agents like immune therapy treatment that has shown promise in only a limited number of breast cancer patients? Will combinations improve efficacy and broaden the group of patients that might benefit? Can we improve the understanding of the chemistry of these agents to reduce toxicity and improve targeting only the cancer cell so that patients can tolerate these treatments better? Again, I remind you if research is an option for you in your cancer journey or someone you know, please consider research. We at NEXT Oncology continue to strive to find better drugs that can fight cancer more effectively.