Research remains an essential part of cancer care. In this blog, we will provide information about recent updates or new approvals as it applies to breast cancer written by Dr. Sharon Wilks.
Dr. Adrienne Waks, breast oncologist at the Dana Farber Cancer Institute, recently presented at the 21st Annual International Future in Breast Cancer East (July 2022). She discussed the current considerations in treatment in patients with HER 2 Neu Positive Breast Cancer. Her presentation was titled, “Candidacy for De-Escalated & Escalated HER 2 directed Therapy in the Curative Setting.”
In this talk, she reviewed the types of current treatments based upon emerging research that are given to patients with stage 1 HER 2 Neu positive breast cancer and those with the same tumor type but who have stage 2 & 3 cancer.
In general, most consider stage I curable with surgery alone when breast cancer is diagnosed. However, it is established that there are some subtypes of breast cancer that surgery alone is not sufficient for long-term survival. In today’s arena, we take for granted the success of HER 2 Neu targeted therapy, but we have to go back quite a long time ago to remember how patients with high risk breast tumors can still do poorly with only an 83% long-term survival rate (17% risk of relapse) in individuals with tumors that are smaller than one centimeter.
What is it about HER 2 Neu disease (and what does that mean exactly?) that influences poor outcomes?
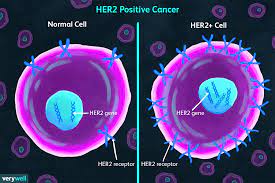
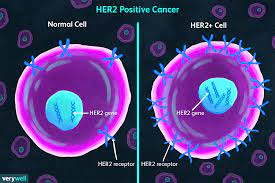
When thinking about this type of breast cancer, what the term ‘HER 2 Neu positive’ implies is an overexpression of this receptor in breast (and other malignancies) cancer. When HER 2 Neu is overexpressed at the cell receptor surface, this produces a powerful signal downstream towards the nucleus which stimulates marked and rapid cell proliferation, angiogenesis, and metastatic potential. Dampening this strong influence in cell growth has been the focus of research long term. As a result, we now have many drugs including Trastuzumab, Pertuzumab, TDM-1, Neratinib, Lapatinib and Trastuzumab Deruxtecan. These are drugs with specific structures that bind to the HER 2 Neu receptor, and we have seen remarkable improved outcomes with less death and less brain recurrences in patients who have these tumors that have received these agents for care.
Knowing that patients who have small cancers still do well overall, research efforts have taken aim to find treatments that offer less short- and long-term side effects to patients at risk with the goal of deriving similar long-term survival as derived from combination regimens that offer more side effects.
Dr. Waks reviewed the APT trial that was updated in 2019 by Tolaney et al. that showed in this group, 7- year recurrence free survival of 97.5%. This is in marked contrast to the 83% historically that did not receive treatment. In the APT trial, patients were treated with Taxol® (Paclitaxel) weekly along with Trastuzumab for 12 weeks then followed by one year of Trastuzumab every three weeks. Newer approaches to reduce toxicity looked at a separated trial known as the ATEMPT trial looking at TDM-1 in place of Paclitaxel. There are ongoing efforts in an ATEMPT 2.0 trial to see again, if using a different agent with less neurotoxicity and possibly less hair loss, can achieve the same great outcomes with less risk of drug toxicity.
In patients with more advanced stages of HER 2 Neu but still with curable cancers (stages 2 & 3), we now have several prospective trials (TRAIN -2 and others) that show us that we don’t need anthracyclines to achieve excellent survival outcomes but with the elimination of Antracyclines (Doxorubicin, Epirubicin), patients are at lower risk for cardiovascular events and leukemia.
Traditionally, Trastuzumab is given for one year after combination drugs to reduce recurrence and though we still don’t have definitive information that the length of this maintenance treatment can be shortened, there are several trials that have shown a similar invasive disease-free survival if the maintenance phase of Trastuzumab is given for six months versus 12 months. Potential candidates for this shortened course are individuals with smaller tumors that are node negative, those who have achieved a Pathologic Complete Remission (PCR) after neoadjuvant therapy or for those who struggle with tolerance of Trastuzumab (which has a good tolerance risk profile, but some rarely struggle to tolerate this agent). This certainly offers comfort to those who can not continue this treatment who have suffered changes in their cardiac testing where it is not safe to continue Trastuzumab for the full year.
Along the lines of who are candidates for ‘de-escalating’ therapy is the hope of finding a key or several key biomarkers that may predict for more favorable outcomes that might give more comfort and confidence that less extensive therapy may be equally effective. Some ongoing research has suggested if ERBB2 gene expression is present, HER 2 enriched subytpes, absence of PI3 Kinase mutations and the presence of TILS (tumor infiltrating lymphocytes) might be predictors of patients who can be spared of more intense forms of treatment. Ongoing research is examining these factors as well as others to help us be smarter about treating patients based upon tumor characteristics.
We have learned so much about how better to cure HER 2 Neu positive breast but even in individuals with small node negative tumors, we still have not achieved a 100% survival rate with current therapies. We still struggle in the decision process of who needs to be given and more importantly, who we can spare, certain treatments. Historical and ongoing research remains essential to find better ways to cure cancer in general and this effort is essential to reduce side effects of treatments. Always consider research if given the opportunity so we can all help find better ways to fight cancer.