Authors:
Marnie Sanders, Vice President, proXimity™, Equicare Health, Inc.
Melanie Morris, Associate Director, Precision Site Network, Precision for Medicine
Maureen Thompson, Chief Technology Officer, NEXT Oncology
This white paper, co-authored by representatives from NEXT Oncology, Precision for Medicine, and Equicare Health, serves as both a case study and a call to action: the future of clinical trials depends on collaboration, technology adoption, and a shared commitment to advancing science.
Key Terminology:
- Discrete field(s): Represents a single, standalone data element with predefined formats or types (e.g., string, integer, date), which help ensure consistency during transfer.
- Data Mapping: The process of defining how data fields from a source system (i.e., EHR) correspond to fields in a destination system (i.e., EDC). It ensures that data is accurately and meaningfully transferred between systems, even if they use different formats, structures, or terminologies.
Introduction
Most clinical trials rely on manual data transcription, taking information from Electronic Health Records (EHRs) and entering it into Electronic Data Capture (EDC) systems through copy-paste workflows or double-entry. Despite years of digital advancements and organizational investments, the data entry process remains slow, error-prone, and burdensome for research sites. Technology solutions such as proXimity™ offer the opportunity for change and standardized efficiencies via direct transfer of structured EHR data into compliant EDC systems, eliminating duplicate entry, transcription errors, manual data entry by up to 80%.
Equicare designed proXimity™ to integrate with—rather than to disrupt— site and sponsor/CRO data workflows, aligning with global data standards, supporting compliance, and deploying without heavy technical lift. Equicare’s commitment to interoperability and data integrity has resulted in a platform that supports seamless, automated data exchange while eliminating manual errors. Its user-centered design prioritizes ease of adoption and operational efficiency. By collaborating with stakeholders across the study ecosystem, Equicare ensures that proXimity™ remains at the forefront of innovation, empowering sites and CROs alike to achieve their goals in accelerating trial timelines, enhancing data quality, and reducing operational burdens.
By working directly with early adopters like NEXT Oncology and CRO partners like Precision for Medicine, Equicare ensures that proXimity™ evolves in line with operational realities on the ground. The platform is built for scalability, ease of use, and measurable ROI.
For NEXT Oncology, a leading site specializing in early-phase oncology research, proXimity™ has proven to be a transformative tool that enhances both efficiency and data integrity. Traditionally, site teams have faced the challenge of managing complex workflows while ensuring data completeness and accuracy, often under tight timelines. With proXimity™, EHR data seamlessly integrates into the EDC system, significantly reducing the time and effort required for manual data entry and reconciliation. This shift has allowed site staff—including Principal Investigators and study teams—to redirect their focus toward critical aspects of trial conduct, regulatory compliance, and, most importantly, patient care. Moreover, direct EHR-to-EDC data transfer has minimized the risk of transcription errors and inconsistencies, reducing the likelihood of audit findings and data queries. NEXT Oncology’s forward-thinking adoption of proXimity™ positions it as a site prepared to meet the increasing complexity and data demands of modern clinical trials, solidifying its role as a preferred partner for CROs and sponsors seeking reliable, efficient, and innovative site collaboration.
As an early adopter of proXimity™ we at NEXT have been using this technology to transfer data to the EDC since 2021. The solution needed was direct transfer of data done historically by data entry specialists, requiring the human transcription of data from various parts of the EHR to the many fields of the EDC. This was costly and time-consuming with the risk of transcription errors. By using the proXimity™ solution, our pharma, biotech, and CRO partners quickly realized, they would have near real time access to better quality data to make informed decisions more quickly. NEXT has benefited from efficiency and cost savings across our data management team.
There have not been many changes in the clinical trial data management space. Most sites still use a system of transcription of information that dates to the scribes in the 15th century. This slowed the transfer of data, and few books were widely distributed. The technology that was transformative then was the Guttenberg printing press. We believe that the use of proXimity™ is a transformative innovation for our sponsors and CROs.
The adoption of recent technologies can be difficult for many organizations and companies. To accelerate this change we mandated all new studies that use Medidata Rave use proXimity™—such that we forced change. This breaks down the usual barriers to change because if the sponsor really wishes to use NEXT for their clinical trials, they must adopt proXimity™. If you dream of the seamless transfer of clinical trial data to those deciding it is safe and effective, proXimity™ is the product for you.
– Dr. Anthony Tolcher, CEO, NEXT Oncology.
Most CROs take an incremental approach to the adoption of innovative technologies, intentionally balancing innovation with established industry practices. Precision for Medicine embraced the opportunity to explore proXimity™, assessing the set-up and degree of integration associated with implementation. A major consideration for Precision was the potential impact to site start-up timelines and other operational impacts that could impede the timely achievement of critical study milestones. By ensuring understanding alignment with both NEXT Oncology and Equicare from the outset, we ensured readiness without compromising speed and potentially accelerating timelines for data cuts, decisions, and locks.
The Precision Data Management team and Equicare work collaboratively for each study to program the EDC, with Precision noting that the Equicare has successfully made the process streamlined and offered support and collaboration to the Precision Data Management teams throughout. What we have learned is that this kind of integration does not require overhauling systems or rebuilding operations; it does however require CROs to be flexible, aligned, and forward-thinking.
By enabling direct EHR-to-EDC transfer, proXimity™ shortens the gap between data collection and data availability in EDC. This automation supports faster interim reviews, cleaner datasets, and stronger site relationships. It is also a win for regulatory alignment— reducing discrepancies that typically trigger queries or delays.
The result? Operational efficiency, less risk, and faster delivery.
CASE STUDY
To illustrate the impact of EHR-to-EDC integration, we present a case study of a blinded, phase I oncology study. Precision was the CRO managing this 10-site trial, 2 of which were NEXT Oncology sites using proXimity™.
Once the EDC, Rave, was finalized, the setup time for proXimity™ was four weeks. All mapping and user acceptance testing (UAT) was handled by the Equicare Health team, ensuring that no additional time or staffing burden was placed on the site, sponsor, or CRO. This streamlined process highlights the efficiency and self-sufficiency of Equicare’s implementation approach.
The graph pictured below provides an overview of mapping efficiency across the various clinical trial data folders and fields for the study. The folders varied in total and discrete field counts, with discrete fields being those eligible for mapping from the EHR. Among them, Cycle Folders (1–6) contained the most data, with 80% of mapped fields. While the End of Treatment/Study, Unscheduled Forms, and Follow-Up forms demonstrated strong mapping rates of 74%, to 79%. This highlights a steady optimization effort in data harmonization across trial phases.
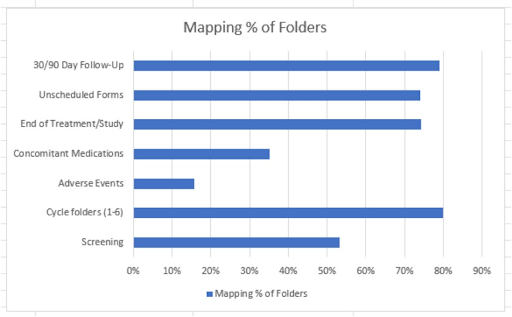
Figure 1. % of mapped fields available to be transferred from the EHR to EDC with the latest version of proXimity™
Discrete data available for mapping included patient demographics (e.g., age, sex, race), vital signs (e.g., heart rate, blood pressure, temperature), and a variety of laboratory results such as chemistries (e.g., electrolytes, liver enzymes), hematology (e.g., hemoglobin, white blood cell count), urinalysis (e.g., protein, glucose), coagulation (e.g., INR, aPTT), and virology panels. Additional structured data included physical exam findings, standardized performance status scores, concomitant medications & adverse events.
Note, proXimity™ is utilized by the data entry specialist at the provider sites to transfer the data once the data has been approved in the electronic medical record. On average, transferring the data takes approximately 18% of the time required for manual data entry, which significantly improves the availability of data in the EDC.
The clustered column chart below provides a comparative visualization of query creation counts between two methods: ‘Manual entry’ and proXimity™’. Each method is represented by a distinct vertical bar, grouped side-by-side for each category of query creation. This layout allows for a clear comparison of the performance or frequency of query creation between the two methods. The chart clearly shows the reduction in the number of raised queries when using proXimity™ vs. manual entry.
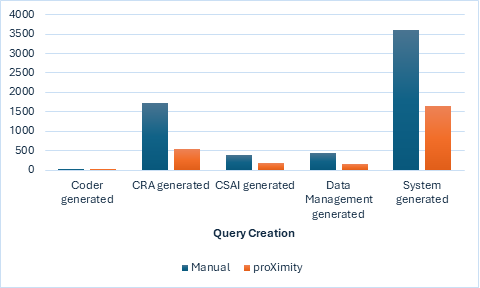
Figure 2. number of queries raised: Manual Data Entry vs. proXimity™ data mapping
Conclusion
Equircare’s development of ProXimity™ represents a large step forward for the industry, uniting CRO operational excellence, site sustainability, and technology innovation. By embracing this technology, we can collectively accelerate timelines, improve data integrity, and reduce burdens across the clinical trial ecosystem.
The collaboration between NEXT Oncology, Precision for Medicine, and Equicare highlights how stakeholders across the clinical trial ecosystem can unite to advance research and improve patient outcomes.
Sponsors, CROs, technology providers, and research sites must work together from study design through execution to ensure alignment, readiness, and mutual understanding. When all parties are engaged and invested in the success of data integration, the result is not only operational efficiency but also stronger partnerships, cleaner data, and faster delivery of life-changing therapies.


