Research remains an essential part of cancer care. In this blog, we will provide information about recent updates or new approvals as it applies to breast cancer written by Dr. Sharon Wilks.
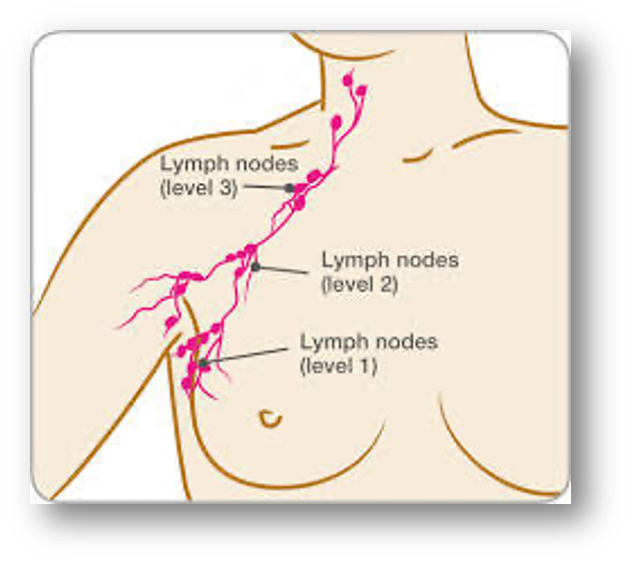
Recently, there was an update on the AMAROS trial that now has 10-year results. In order to have perspective on this report, it is important to know that in the past if someone had undergone testing of the sentinel lymph node and it was positive for malignancy, it was commonly felt necessary to have the patient undergo extensive lymph node removal, a procedure referred to as an axillary node dissection. (It is also important to note that the sentinel lymph node is a key node that is identified prior or during the definitive breast surgery. If cancer cells are found, there is concern that there may be more nodes deeper that should be removed; while in contrast, if it is negative for cancer cells, then it is more reassuring that deeper nodes are not likely to have cancer.) Sentinel node dissection is so named because it is felt that this is the node physiologically that is the first stop for cancer cells to travel to when cancer cells are attempting to spread to other places in the body.
Thanks to observations in older studies, many individuals found to have one sentinel node positive after careful axillary dissection were only found to have one to two additional nodes with cancer present and greater than 60-70%, when axillary dissection was done, many were found to have no malignancy in the remainder of nodes. This observation raised the question of the necessity of removing more nodes if indeed the odds were that the untested remaining nodes were likely not to have cancer. Could we spare the patient additional surgery in this setting? If we could do less surgery, could this lead to less long-term complications from such an extensive surgery which often included arm and chest pain, lymphedema and limited range of motion?
An update in the Journal of Clinical Oncology (JCO) vol. 41 issue 12 in April 2023, Radiotherapy or Surgery of the Axilla After a Positive Sentinel Node in Breast Cancer: 10-Year Results of the Randomized Controlled EORTC 10981-22023 AMAROS Trial reported by Bartels, SAL et al. provided an update at 10 years on alternative management of the axilla when a sentinel node is found to be positive for patients with clinical evidence of early stage breast cancer.
This study is often referred to as the AMAROS Trial. This update confirmed that there was no advantage to axillary dissection in these patients with similar axillary recurrences and overall survival as well as locoregional control. The aim of the study was to verify that there was no advantage to surgery over use of radiation to the axilla instead. Typically, if nodes are found involved (particularly if it is four or more nodes involved), post-operative radiation is required anyway so one of the primary study goals was to confirm that there was not a better outcome with the addition of additional axillary surgery in this setting.
Study Details:
The details of this study indicated the following: This was a Phase 3 open-label multicenter noninferiority trial including 4,806 patients who underwent sentinel lymph node biopsy. Of this group, 1,425 patients were found to have node-positivity of the sentinel node and it was this group who were randomly assigned to either complete Axillary Lymph Node Dissection (ALND) versus Axillary Radiation Therapy (ART). Patients were deemed eligible for participation in this study if they were found to have clinically small tumors (T1-T2 which is equivalent to tumors less than five centimters in size) that were clinically node negative but on sentinel lymph node biopsy were found to have positive involvement of that node with breast cancer.
In this situation, the patients were randomly assigned to either ALND or ART. ALND involved removal of anatomic level 1 & level 2 nodes and less than or equal to 10 nodes were removed. ART was 25 fractions of 2 Gy to all 3 levels of the axilla as well as the medial part of the region known as the supraclavicular fossa.
Adjuvant radiation was given to patients in the ALND assigned arm if the patients were found to have four or more positive nodes on dissection.
The primary endpoint of the study was to determine the risk of tumor recurrence in the axillary or regional nodes and secondary endpoints included the survival including overall (OS) and disease-free survival (DFS) as well as the development of lymphedema, shoulder mobility changes and quality of life (QOL) between the two groups. Note that enrollment of these patients was from February 2001 to April 2010.
Findings of the study:
Axillary recurrences were reported in 7 or 0.9% of patients in the ALND compared to 11 or 1.6% of the ART group. There were 104 deaths (14%) in the ALND group and 112 (16.4%) deaths in the ART group. The 10-year OS was 84.6% in the ALND group and 81.4% in the ART group. Ten-year DFS was 75% in the ALND and 70.1% in the ART group.
As for complications of therapy and quality of life (QOL), 44.2% of patients reported lymphedema during some time post treatment in the ALND group compared to 28.6% in the ART group. The time frame for the highest rate of onset was at year one of follow-up. Shoulder mobility was similar in both groups.
Conclusions of study:
The AMAROS Trial demonstrated after a median follow-up of 10 years that both ALND & ART provided excellent locoregional control in patients with clinically node negative and tumors </=5 cm who were found to have a positive sentinel lymph node. There were no differences in the overall and disease-free survival.
Furthermore, this 10-year update confirmed a significantly lower lymphedema rate after ART at all time points and overall, no differences in shoulder mobility or overall QOL. The study confirmed, like other similar trials, the very low long-term rate of axillary recurrences.
There were more second primary cancers reported in the ART group compared to the ALND group. Previous studies had shown a small excess risk of second primary cancers in or near the radiation fields. Though clearly this higher rate of new cancers is concerning, overall the 10-year results were very reassuring for the excellent long-term local and regional control and overall good QOL of patients after ART amongst patients with early stage of breast cancer and a positive sentinel node on biopsy.
Clinical research continues to provide answers to ongoing questions. Currently, we have data that clearly demonstrates that overall breast cancer survival rates continue to improve but this study reviewed here is an example of the importance of looking at ways to reduce short- and long-term complications from treatment that is providing cure.
Though this is an important finding, we still see in this trial that some patients with early-stage breast cancer still died from cancer, and we have some concern over a higher rate of new primary cancers reported in the ART group. This reveals the need of continuance of research. At NEXT Oncology, we are involved with evaluating new treatments in hopes of improving survival rates, and hopefully, will lead to measures to improve tolerance of these treatments that can reduce suffering amongst patients with cancer. If you or someone you know have an opportunity to participate in a clinical research study, we would encourage you to consider this form of care. It can potentially lead to an improvement in survival and quality of life post treatment for cancer.
