Research remains an essential part of cancer care. In this monthly blog, we will provide information about recent updates or new approvals as it applies to breast cancer written by Dr. Sharon Wilks.
Hormone Positive Breast (HR+) cancer defined by biopsy results of having a breast cancer staining positive for either the Estrogen Receptor (ER) or Progesterone Receptor (PR), or both, is the most common form of breast cancer diagnosed today. Recent estimates suggest that at least 70% of patients with newly diagnosed breast cancer have HR positive breast cancer.
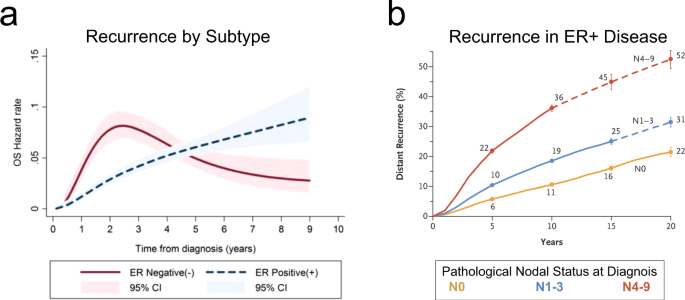
Historically, this subtype of breast cancer was felt to confer a much more positive breast cancer survival rate than other breast cancer subtypes (i.e., Triple Negative or HER 2 Neu positive breast cancer). Emerging reports including data presented at the recent American Society of Clinical Oncology (ASCO) meeting in June 2022 relay a sobering observation in that these patients are more likely to experience continued lifelong risk of relapse after five years of survival. Current estimates suggest that as many as 1% of patients with HR+ breast cancer per year recur with many observed to have distant sites of recurrence such as metastases in the bone, liver, lung and other organs.
With this discovery of latent relapse in the previously so called ‘favorable risk’ breast cancer, there have been many ongoing efforts in research to augment effects of endocrine blockade in patients with HR+ disease. It is now understood that women with large breast tumors or that have node positive disease (particularly greater than three nodes) continue to experience high risk of late recurrences beyond 10-20 years of survivorship.
For many years, Tamoxifen, a common hormone blocker agent known as a SERM (Selective Estrogen Receptor Modulator) was used for five years. In a recent review at ASCO, a review of published reports in the last 8-12 years have shown that women who only took this agent for five years were at higher risk for distant recurrences. Most individuals who are considered for this treatment are premenopausal (meaning the individual still has normal estrogen production from the ovaries). When studies known as ATTOM & ATLAS were reviewed (these studies randomized patients to stop Tamoxifen use at five years versus continuing Tamoxifen until 10 years) showed striking reduction of late recurrences in those individuals who took Tamoxifen longer than five years.
Women who are older and postmenopausal (no longer have ovaries in place or have loss of estrogen production from the ovaries through normal aging), are commonly considered to be given Aromatase Inhibitor therapy (AI) if their cancers are HR+. The concept of value of these agents’ effect is that a natural enzyme that converts natural androgens into estrogen-this enzyme is known as Aromatase is that the AIs work to inhibit estrogen production in the older female by blocking the Aromatase from conversion.
Early studies of AI therapy in the postmenopausal setting had shown clear superior benefit over use of Tamoxifen in this group. However, now that we realize some patients are at risk for late recurrences, there is an interest to determine if extended therapy – that is use of AI or ET longer than five years may be of value to prevent late recurrences.
At the recent ASCO 2022, Dr. Barbara Pistilli gave an overview of this concept with a talk titled, “Optimizing Duration of Endocrine Therapy.” In this presentation, she reviewed several trials that are well recognized research trials including nine studies referred to as ATTOM, ATLAS, MA17-R, NSABP-B42, IDEAL, ABCSG, DATA & GIM-4. These studies had looked at duration of therapy greater than five years for either Tamoxifen alone, Tamoxifen followed by AI use.
In review of these studies, we have some new guidance on how to determine who may or may not need or benefit from extended therapy:
- Young premenopausal women with small tumors (less than 1-2 cm in size) that are node negative may not need long term ET.
- Young women with large tumors or node positive tumors should continue Tamoxifen if they remain premenopausal. Some of these patients may be better served by being administered adjuvant treatment with Ovarian Function Suppression (OFS) +/- Tamoxifen or an AI (particularly if they are diagnosed under 40 years of age).
- Older women with Node positive breast cancer may benefit from longer than five-year use of AI, and generally, most agree that this can be as short as 7-8 years total of AI but some clinicians extend this therapy if the patient is tolerating treatment well up to five years.
Thanks to research, we recognize these treatments do pose a risk including thromboembolic events and uterine cancer in those who take Tamoxifen (this risk continues if one takes the drug longer than five years) and bone fractures and bone pain (arthralgias and myalgias) with AI use.
Recently, the FDA approved addition of an agent known as a CDK4/6 inhibitor known as Abemaciclib in the early stage setting for women with large breast tumors and or significant node involved ranging from 1-4+ cm with modestly elevated Ki67 of 20% or more. This approval was based upon a study known as Monarch E that showed a significant risk reduction of recurrent breast cancer when added to endocrine therapy.
There are still many unmet needs in this kind of breast cancer and other novel strategies are ongoing to optimize dosing and reduce side effects and to improve breast cancer survival rates not only in the first five years of survivorship but beyond.
We at NEXT Oncology are studying new agents in HR+ breast cancer. We urge you to consider clinical trials if you have access to them. Only with research can we provide a better outcome in cancer survival and quality of life for the cancer survivor.
